“Life cycle Approach to Process Validation” Part-1.
Author: Sanjeev Kumar Singh
Deputy General Manager - Corporate QualityAssurance at Mylan Laboratories Limited
www.gmpviolations.com
GMP News, GMP guidelines, GMP Violations, GMP warnings, GMP Trends. A Public Health Global News Portal. (This story has not been edited by GMP Violations staff and is auto-generated from a syndicated feed.) Disclaimer: The Logos/Images & content posted here are belongs to respective to Authority / owners of firm. The Article posted under public health importance news.
“Quality has to be built-in into the process
and cannot be relied on the testing of final product”
Definition of Process validation:
“The
collection and evaluation of data, from the process design stage through
commercial production, which establishes scientific evidence that a process is
capable of consistently delivering quality product.”
“Process Validation (PV)” concept in pharmaceutical industry
was first proposed by USFDA officials, namely, Ted Byers and Bud Loftus in
mid-1970, who believed that, introduction of PV will help to improve the
quality of pharmaceutical products. Though, initial proposal was in direct
response to several problems in the sterility of large volume
parenteral market and first validation activities were focused on the
processes involved in manufacturing these products, which subsequently expanded
to associated processes including environmental control, media fill,
equipment sanitization, purified water production, process equipment’s
software, methods and processes etc.
First
USFDA draft guidance document on “PV” came into existence in 1987. The
objective of the guidance was to bring awareness among the pharmaceutical
sphere in describing the agency’s thinking so as to produce safe and consistent
quality of medicines to patients. As the new concepts of “Quality by
Design/(QbD) ” emerge with the inception of ICH guidelines namely, Q8, Q9, Q10
& Q11 , which led to introduction of enhanced approach of validation as
“Life Cycle Approach to Process Validation” explicitly outlined in the PV
guideline released by USFDA in January 2011.
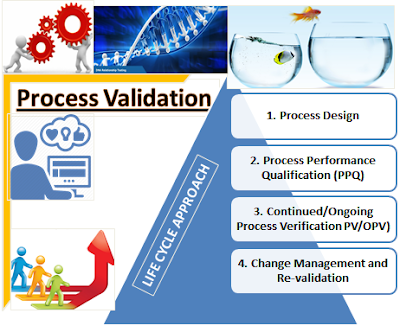
PROCESS
VALIDATION – LIFE CYCLE APPROACH:
4
STAGE PROCESS:
The current life cycle approach to “Process Validation”
divulges that PV is a journey and not a one-off event of just completing the 3
PV runs. All phases in the life of a product from the initial development
through marketing until the product’s discontinuation are called life cycle
approach to PV.
Enhanced PV model is a science and risk based approach
aligning with the principles of “Quality by Design” articulated in ICH
guidelines Q8, Q9, Q10 & Q11. A lifecycle approach is applied linking
product and process development, validation of the commercial manufacturing
process, and management of the process within the change management system to
make sure maintenance of the process in a state of control during routine
commercial production” is defined below:
Stage-1: Process Design: The commercial manufacturing process is defined during this
stage based on knowledge gained through development and scale-up activities.
Stage-2: Process Performance
Qualification (PPQ): The
process design is evaluated to determine if the process is capable of
reproducing commercial manufacturing.
Stage-3: Continued/Ongoing Process
Verification PV/OPV):
Ongoing
assurance gained during routine production that the process remains in a state
of control.
Stage-4: Change Management and
Re-validation:
Ongoing assessment of the process as part of change
management system to ensure the re validation and maintain continuum validated
state of control.
Author: Sanjeev Kumar Singh
Deputy General Manager - Corporate QualityAssurance at Mylan Laboratories Limited
Keywords: Process validations, Quality by Design, performance qualification, change management.
www.gmpviolations.com
GMP News, GMP guidelines, GMP Violations, GMP warnings, GMP Trends. A Public Health Global News Portal. (This story has not been edited by GMP Violations staff and is auto-generated from a syndicated feed.) Disclaimer: The Logos/Images & content posted here are belongs to respective to Authority / owners of firm. The Article posted under public health importance news.






















0 comments:
Post a Comment