When you buy prescription and
over-the-counter medicines, sunscreens or vitamin supplements, you need
important information to help you make an informed choice.
Medicine labels tell you what you are buying, what the medicine can do for you and how to use it.
What is changing under the new labelling rules?
We are changing medicine labels to make
important information about your medicine easier to find. These changes
are the result of many years of consultation - they bring Australian
medicine labels up to date with international best practice.

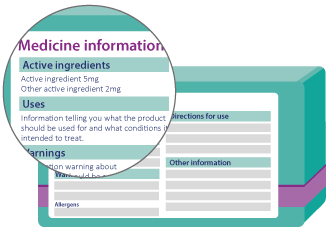
Active ingredients will be easier to find
The active ingredient is the substance in the medicine that makes it work.
You probably know of paracetamol, ibuprofen or insulin - all of these substances are active ingredients. Under the new labelling rules active ingredients need to be more prominent. You will usually be able to find them below or next to the product name on the front of the medicine pack. Active ingredients will often be in a larger print size on the front label to make them easier to read.
Make sure to look for the active ingredients on your medicine labels so you know what you are taking.
You probably know of paracetamol, ibuprofen or insulin - all of these substances are active ingredients. Under the new labelling rules active ingredients need to be more prominent. You will usually be able to find them below or next to the product name on the front of the medicine pack. Active ingredients will often be in a larger print size on the front label to make them easier to read.
Make sure to look for the active ingredients on your medicine labels so you know what you are taking.

Medicine information will be clearer
Most over the counter medicines will have critical health information
in distinctive tables to help you use your medicine safely. Over the
counter medicines are medicines that you buy without a prescription.
The new rules mean that critical health information will always be displayed in a consistent order and will be easy to recognise.
Always check the critical health information before you take your medicine.



The new rules mean that critical health information will always be displayed in a consistent order and will be easy to recognise.
Always check the critical health information before you take your medicine.
More information on the label
Under the new rules more substances that could cause an allergic
reaction will need to be included on labels. These substances include
crustacea, fish, eggs, soya, milk and tree nuts.
For non-prescription medicines this information will be on the label. For prescription medicines this information must appear on the label or in the Consumer Medicine Information leaflet with a prompt on the pack.
For non-prescription medicines this information will be on the label. For prescription medicines this information must appear on the label or in the Consumer Medicine Information leaflet with a prompt on the pack.

More room for important information
The new rules include a minimum space for dispensing labels. These
are the labels that pharmacists stick on prescription medicines with
information from your doctor.
This space makes it easier for the pharmacist to attach the dispensing label without covering up other important information.
This space makes it easier for the pharmacist to attach the dispensing label without covering up other important information.
Why are medicine labels changing?
Labelling requirements for Australian
medicines are being updated after many years of consultation with
industry, health professionals and the community. The changes help bring
Australian medicine labels up to date and align them with international
best practice. They will help Australians to make more informed choices
about their medicines and use them more safely.
When are labels changing?
The new labelling rules took effect from 31
August 2016. There is a four year transition period to allow medicine
manufacturers time to update their labels and to sell their existing
stock. This means that after 31 August 2016 you may start seeing updated
medicine labels, but you could still see older labels as well. During
the transition period both versions are acceptable - manufacturers need
to meet either the old or the new rules.
From 1 September 2020 all medicine labels will need to meet the new rules.
What other information is on my label?
Labels have all sorts of useful information on them. Next time you look at a medicine keep an eye out for:
AUST R and AUST L numbers
Medicines sold in Australia will have either an AUST R or an AUST L number (but not both).
AUST R numbers
AUST R medicines (also known as
registered medicines) are assessed for quality, safety and effectiveness
before they can be sold. They include all prescription-only medicines
and many over-the-counter medicines.
An AUST R number means the medicine is more tightly controlled and regulated.
AUST L numbers
AUST L medicines (also known as listed
medicines) are lower risk self-medication products. They are used for
minor health problems and are less regulated than AUST R.
Listed medicines include fish oils, multivitamins and herbal and homoeopathic products.

Storage conditions
Labels have to tell you how to store a product - some medicines lose their effectiveness if not stored correctly.

Expiry date
This is similar to the use-by date on
food. Medicines should never be used after their use-by date - they can
lose their effectiveness or even become unsafe.

Batch number and company address
The batch number and name and address of the supplier can be used to trace a medicine if a problem is found.
























0 comments:
Post a Comment